Chemistry, precisely
We scale metal-organic frameworks to reduce the negative impact of chemical products and processes on human health and the environment.
MOF solutions designed to tackle the greatest challenges facing global industries now and in the future
We help our customers achieve their innovation and sustainability objectives by improving the efficiency of the critical industrial products and processes powering global growth.

MOF market leaders
We are the market leader in metal-organic frameworks (MOFs), a transformative precision chemistry platform that can capture hazardous chemicals with incredible precision.

Speed meets scale
Our world-class platform integrates MOFs into existing products and processes, merging chemistry innovation with manufacturing at industrial scale.
Solving Industrial Challenges
Precision chemistries, at scale



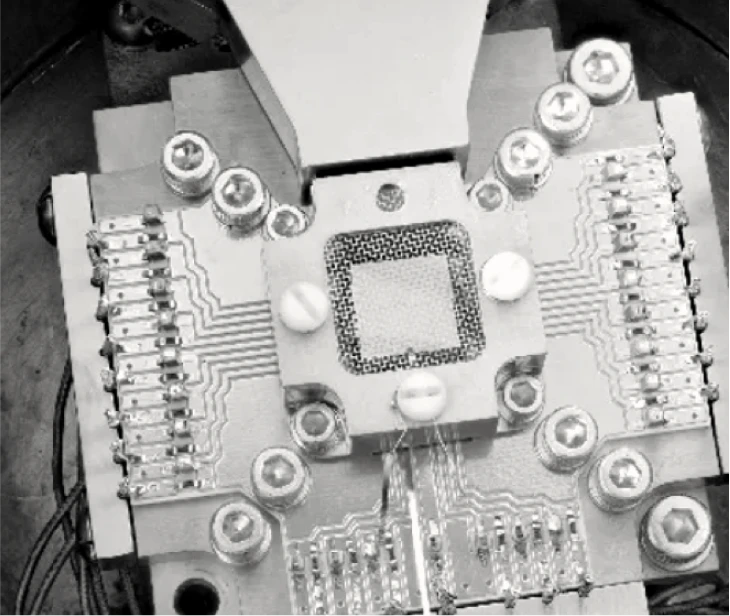
Next-Generation Chemical Delivery
Enabling our digital future
The global semiconductor industry trusts ION-X® delivery systems to transport critical, yet hazardous electronic materials to the tools fabricating chips one atomic layer at a time.
Powered by
ION-X®
The future of chemical protection
Protection for mission critical environments
SENTINEL™ filtration enables next-level chemical protection and decontamination in a single material, transforming how war fighters and front line workers are protected from chemical threats.
Powered by
SENTINEL™


Reducing emissions sustainably
Powering a net zero future
Numat’s SENTINEL™ filtration shields our built environment from industrial emissions by precisely capturing and separating hard-to-abate toxic and greenhouse gases.
Powered by
SENTINEL™

Precision chemistries at scale
Our facilities
Today, we operate the world’s only ISO 9001:2015 certified facility for the final assembly of MOF products sold into leading edge industrial applications.
In 2024, Numat is relocating its headquarters to a former foundry in Chicago, being repurposed to a state-of-the art nanofabrication and manufacturing center of excellence.
Industries We Serve
Semiconductor
Life cycle management of critical electronic materials

Extreme Environments
Next-generation chemical and biological protection solutions

Energy
Decarbonizing chemical separations and reducing emissions intensity

Careers at Numat
Be part of something bigger
Numat is built on a future-driven culture, powered by an extraordinary team. We are unified by a shared set of values and a passion to drive industrial efficiency through chemistry innovation.